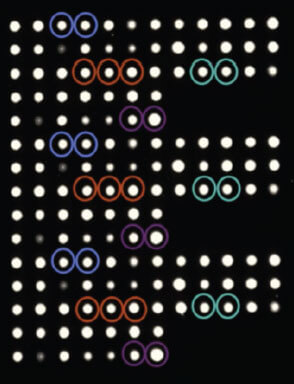
“Together PathogenDx and Prevenio are bringing safety innovations to market that will save significant time and cost in the process of identifying and addressing foodborne health risks. When combined, our unique technologies represent a leading-edge model of food safety that encompasses rapid detection and pinpoint intervention. Instead of working backwards from the point of harm, producers and regulators can now adopt more forward-looking, preventative food safety processes.”
John Meccia
President and CEO, Prevenio