Protectx
A safer future isn’t just
something we wish for.
We’re creating it.

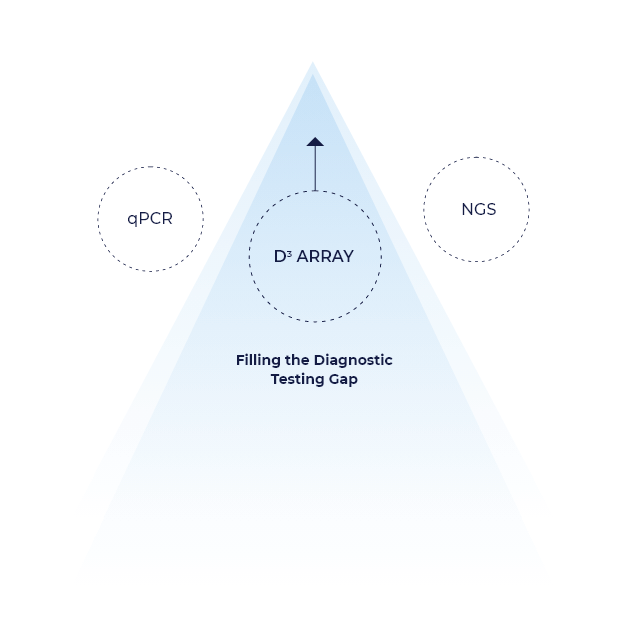
Even with two well-established diagnostic technologies, there are instances where the sensitivity and multiplex limitation of qPCR are insufficient or the cost and timeliness of NGS are problematic. The D3 Dynamic Dimensional Detection Array can fill this gap, ideally suited to simultaneously identify up to 100 targets and delivering single-gene copy sensitivity with accompanying economies and speed.







|
Product Safety Solutions |
Description |
Clinical Safety |
Food Safety |
Agricultural Safety |
Pathogens |
|
|
Poultry/Food Safety Solutions |
||||||
|
|
Rapidly detects and quantifies Salmonella Spp. in unenriched sample rinsates and detects regulated Serotypes, down to 1 CFU/test portion, in both chicken and turkey. |
|
Click for list of specific pathogens
Detection & Quantitation: Salmonella Spp |
|||
|
|
(PTM Certification 092001) Performance Tested Method Status by the AOAC Research Institute for the multiplex detection of Salmonella spp., Listeria spp., and L. monocytogenes without an enrichment step. 4 different commonly used food surfaces. The assay is validated down to 1 CFU without the need for sample enrichment. Surface & Air SARS CoV-2 detection |
|
Listeria spp, Listeria monocytogenes and Salmonella on rubber, plastic, stainless steel and sealed concrete to 1 CFU/gram. |
|||
|
Agriculture Safety Solutions |
||||||
|
|
Detects bacterial and fungal species For Cannabis and Hemp testing, this assay detects bacterial and fungal species in a single well and a single test. Dramatically reduces hands-on steps and cost with results in 6 hours or less. Overnight enrichment is not necessary and optional dependent on state-level testing regulations. Additional reagents available for LIVE organism testing. |
|
Click for list of specific pathogens PTM certified for STEC, Salmonella spp, Aspergillus Flavus, Aspergillus Fumigatus, Aspergillus Niger, and Aspergillus Terreus. Customizable to test for other pathogens including E. coli (pathogenic and non pathogenic), Listeria monocytogenes, Listeria spp, Campylobacter spp, Staphylococcus aures, and Pseudemonas aeruginosa. |
|||
|
|
Detects fungal species |
|
Click for list of specific pathogens Aspergillus Flavus, Aspergillus Fumigatus, Aspergillus Niger, and Aspergillus Terreus |
|||
|
|
Detects bacterial species |
|
Click for list of specific pathogens STEC, Salmonella spp, E. coli (pathogenic and non pathogenic), Listeria monocytogenes, Listeria spp, Campylobacter spp, Staphylococcus aures, and Pseudemonas aeruginosa |
|||
|
|
Quantification for fungal pathogens QuantX Fungal is a semi-quantitative assay for fungal species in total yeast and mold (TYM), providing results in a single shift. QuantX Fungal delivers unrivaled quantitative analysis ease for labs, expanding your client regulatory offerings (and ROI) with one simple sample prep (automated or manual) across all of your Cannabis testing assays. |
|
Multiple Yeasts and Molds
Alternarium Candida Albicans Golovinomyces Botrytis Cladosporium |
|||
|
|
Quantification for bacterial pathogens QuantX Bacterial is a semi-quantitative assay combining analysis of four broad class indicators plus species specific detection within each broad class. QuantX Bacterial delivers unrivaled ease of analysis for labs, expanding your client regulatory offerings (and ROI) with the added benefit of one simple sample prep (automated or manual) across all of your Cannabis testing assays. |
|
Multiple Bacterial Class Indicator Organisms
One test delivering semi-quantitative analysis* of:
|
|||
|
|
Detects Hop Latent Viroid and 8 other plant viruses in a single test, in three hours or less. Sample prep reagents included with kit. |
|
Click for list of specific pathogens
Hop Latent Viroid (HLVd); Lettuce Chlorosis Virus (LCV); Cannabis Cryptic Virus (CanCV); Beet Curly Top Virus (BCTV); Tobacco Streak Virus (TSV); Arabis Mosaic Virus (ArMV); Alfalfa Mosaic Virus (AMV); Tobacco Mosaic Virus (TMV); Cucumber Mosaic Virus (CMV)
|
|||
|
Clinical Safety Solutions |
||||||
|
|
Detects 26 pathogens associated with Urinary Tract Infections and 12 Antibiotic Resistance Genes in a single test |
|
Click for list of specific pathogens Acinetobacter baumannii, Aerococcus urinae, Citrobacter (freundii, koseri), Enterobacter (aerogenes, cloacae), Enterococcus (faecalis, faecium), Escherichia coli, Klebsiella (oxytoca, pneumoniae), Morganella morganii, Mycoplasma (hominis, genitalium), Proteus (mirabilis, vulgaris), Providencia stuartii, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus (aureus, saprophyticus), Streptococcus agalactiae, Ureaplasma (parvum, urea), Candida (albians, glabrata) |
|||
|
Environmental Safety Solutions |
||||||
|
|
"Early warning" Surface & Air pathogen detection Environmental Screening test for the presence or absence of 30+ bacterial and/or fungal pathogens as well as broad class indicators. This assay can be utilized with multiple sample collection types, including but not limited to food, cannabis and agriculture products, environmental or carcass swabs, air sampling and water sampling. |
|
Over a dozen bacterial and fungal pathogens
Bacterial Organisms |
|||
|
|
(PTM Certification 092001) Performance Tested Method Status by the AOAC Research Institute for the multiplex detection of Salmonella spp., Listeria spp., and L. monocytogenes without an enrichment step. 4 different commonly used food surfaces. The assay is validated down to 1 CFU without the need for sample enrichment. Surface & Air SARS CoV-2 detection |
|
Listeria spp, Listeria monocytogenes and Salmonella on rubber, plastic, stainless steel and sealed concrete to 1 CFU/gram. |
|||
|
Live vs. Dead |
||||||
|
All Products |
Isolation of "live" DNA for pathogen detection Sample preparation to prevent non-viable cells from being amplified preventing false positives. This product is a supplement to current sample preparation methods. |
|
|
|||